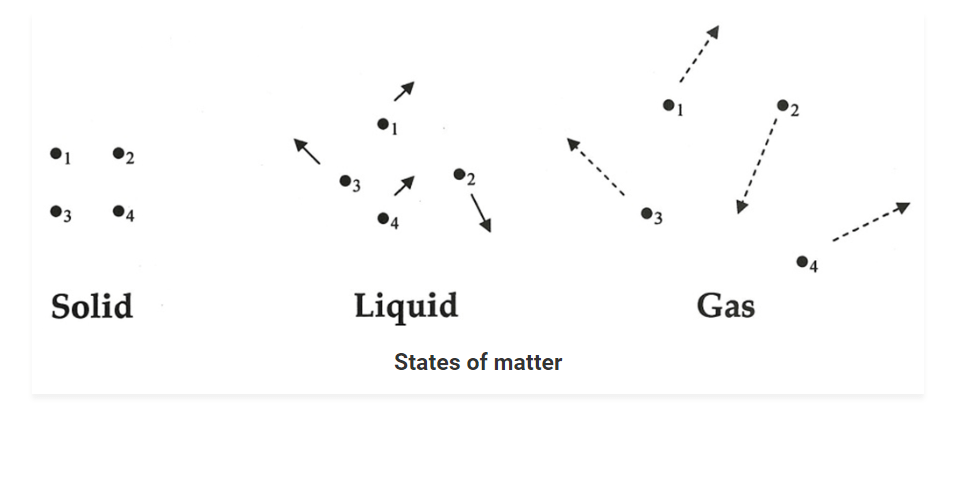
STATES OF MATTER
• It has been observed that matter exists in nature in different forms. Some substances are rigid and have a fixed shape like wood and stone; some substances can flow and take the shape of their container like water, while there are forms of matter that do not have definite shape or size such as air.
• Thus, the matter can be classified into different categories based on the physical properties exhibited by them and the states in which they exist; these are called states of matter.
• Following are the basic three states of matter:
- Solid
- Liquid
- Gas
• Apart from the above mentioned three, there are 2 more states of matter which we do not see in our everyday life. They are
- Plasma
- Bose-Einstein condensate
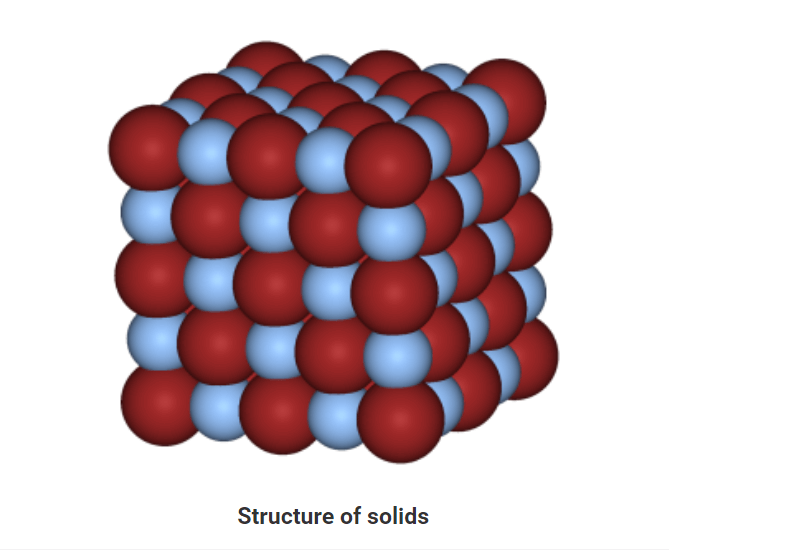
Solids
• In solids, particles are tightly or closely packed.
• The gaps between the particles are tiny and hence it is tough to compress them.
• Solid has a fixed shape and volume.
• Due to its rigid nature, particles in solid can only vibrate about their mean position and cannot move.
• Force of attraction between particles is adamant.
• The rate of diffusion in solids is very low.
• An example of solids: solid ice, sugar, rock, wood, etc.
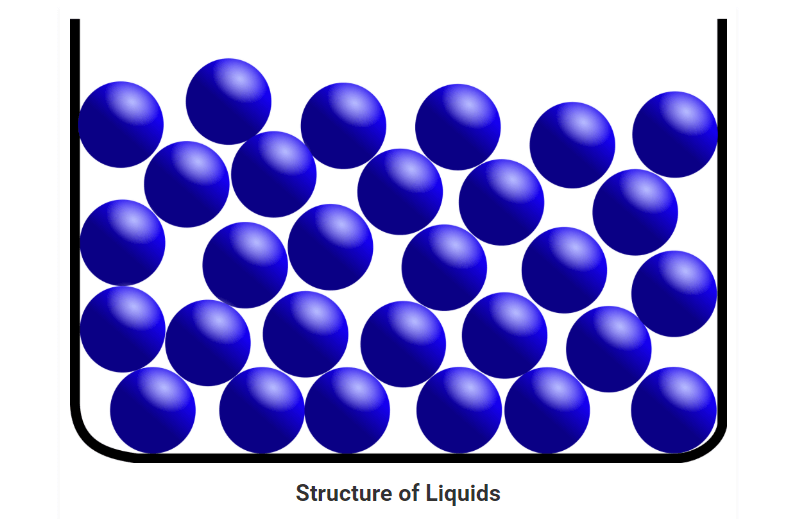
Liquids
• In a liquid state of matter, particles are less tightly packed as compared to solids.
• Liquids take the shape of the container in which they are kept.
• Liquids are difficult to compress as particles have less space between them to move.
• Liquids have fixed volume but no fixed shape.
• The rate of diffusion in liquids is higher than that of solids.
• Force of attraction between the particles is weak than solids.
• Example of a liquid state of matter: water, milk, blood, coffee, etc.
Gases
• In gases, particles are far apart from each other.
• Force of attraction between the particles is negligible and they can move freely.
• Gases have neither a fixed volume nor a fixed shape.
• The gaseous state has the highest compressibility as compared to solids and liquids.
• The rate is diffusion is higher than solids and liquids.
• The kinetic energy of particles is higher than in solids and liquids.
• An example of gases: air, helium, nitrogen, oxygen, carbon dioxide, etc.
Plasma
• Plasma is a not so generally seen form of matter. Plasma consists of particles with extremely high kinetic energy. Electricity is used to ionize noble gases and make glowing signs, which is essentially plasma.
• Superheated forms of plasma are what stars are.
Bose-Einstein Condensates
• Discovered in 1995, Bose-Einstein condensates were made with the help of the advancements in technology.
• Carl Weiman and Eric Cornell cooled a sample of rubidium with the help of magnets and lasers to within a few degrees of absolute zero.
• At the said temperature, the motion of the molecules becomes negligible. As this brings down the kinetic energy, the atoms no longer stay separate but they begin to clump together. As the atoms join together they form a super-atom.
• Light slows down as it passes through a BEC helping scientists to study more about the nature of light as a wave and particle.
• BEC’s also show properties of a superfluid which implies, it flows without friction.
Change in states of matter
Solid state → Liquid state
• On increasing temperature of solids, the kinetic energy associated with the particles of the matter increases. As the kinetic energy of the particles increases they begin to vibrate with a higher frequency. Therefore the force of attraction between the particles reduces.
• As a result particles get detached from their position and begin to move freely. At this state solids undergo a phase change to form liquids. This process is known as melting.
Liquid state → Gaseous state
• When adequate amount of heat is supplied to liquids, the particles of the liquid begin to move with greater speed. At a particular temperature the particles possess enough energy to break away from the force of attraction that exists between the particles.
• At this point the particles undergo phase change and exhibit vapour phase as well as liquid phase. The point at which this happens is called boiling point. This process is called as evaporation.
• Sometimes solids get converted into gaseous state directly and the phenomenon is called as sublimation.
• All forms of matter have the capability to change from one state of matter to another. The change is mainly due to the difference in the forces that hold the particles together. It can be concluded that temperature and pressure play an important role in determining the state of a matter i.e. weather it is in solid, liquid or gaseous state at a given set of temperature and pressure.
ALLOTROPES OF CARBON
• The phenomenon by which an element can exist in more than one physical state is called allotropy.
• The allotropes of carbon can be categorized into two:
- Amorphous Carbon Allotropes
- Crystalline Carbon Allotropes
Allotropes of Carbon
• Carbon with atomic number 6 and represented by the symbol ‘C’ in the periodic table is one of the most influential elements we see around us. Carbon is one of the elements which shows allotropy. The allotropes of carbon can be either amorphous or crystalline (Diamond, Graphite).
• Carbon due to its capability of having variable oxidation states or coordination number makes carbon one of the few elements to have multiple numbers of allotropic forms. Carbon’s ability to catenate is another contributing factor. Thus, it leads to the formation of various allotropes of carbon.
Different Allotropes of Carbon
Diamond: It is extremely hard, transparent crystal, with the carbon atoms arranged in a tetrahedral lattice. This allotrope of carbon is a poor electrical conductor and an excellent thermal conductor.
Lonsdaleite: These are also called hexagonal diamond.
Graphene: It is the basic structural element of other allotropes, nanotubes, charcoal, and fullerenes.
Q-carbon: These carbon allotropes are ferromagnetic, tough, and brilliant crystal structure that is harder and brighter than diamonds.
Graphite: It is a soft, black, flaky solid, a moderate electrical conductor. The C atoms are bonded in flat hexagonal lattices (graphene), which are then layered in sheets.
Linear acetylenic carbon (Carbyne)
Amorphous carbon
Fullerenes, including Buckminsterfullerene, also known as “buckyballs”, such as C60
Carbon nanotubes: Allotropes of carbon with a cylindrical nanostructure.
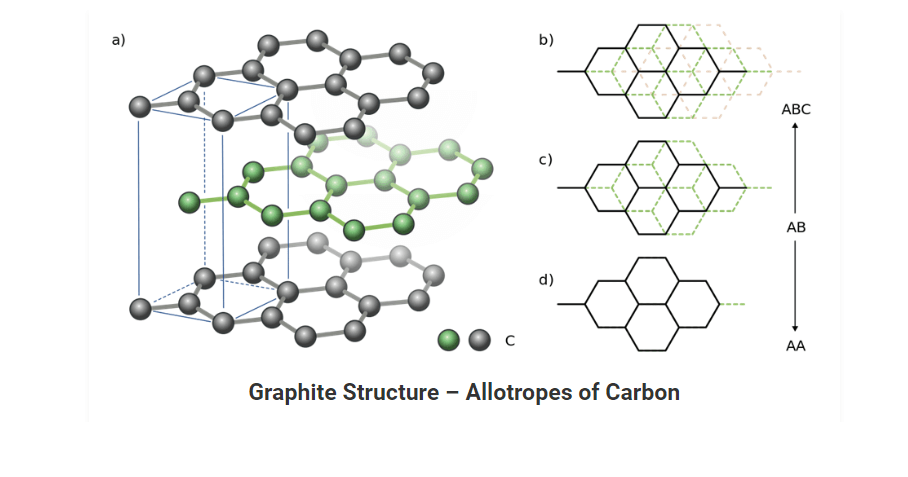
Graphite
• It is also a pure form of carbon. This allotrope of carbon is composed of flat two dimensional layers of carbon atoms which are arranged hexagonally.
• It is a soft, black and slippery solid. This property of graphite persists because it cleaves easily between the layers.
• In each layer, each C atom is linked to three C atoms via a C-C covalent bond. Each carbon here is sp2 hybridized. The fourth bond is formed as a pi bond. Since the π-electrons are delocalized, they are mobile and can conduct electricity.
Properties of Graphite:
• Since the layers are stacked over each other, this carbon allotrope can act as a lubricant.
• It also has metallic lustre which helps in the conduction of electricity. It is a very good conductor of both heat and electricity
• One of the most important properties of graphite is that it is used as a dry lubricant for machines at high temperature where we cannot use oil.
• Graphite is used to make crucibles which have the property that they are inert to dilute acids as well as to alkalis.
• Note: In comparison to diamond, Graphite is thermodynamically more stable.
Structure of Carbon Allotrope (Graphite):
• Graphite has a unique honeycomb layered structure. Each layer is composed of planar hexagonal rings of carbon atoms in which carbon-carbon bond length within the layer is 141.5 picometers.
• Out of four carbon atoms three forms sigma bonds whereas the fourth carbon forms pi-bond. The layers in graphite are held together by Vander Waal forces.
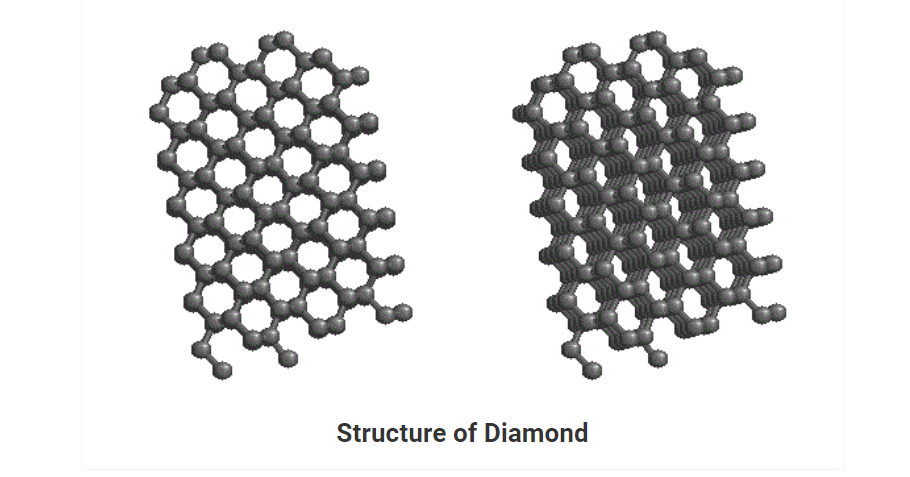
Diamond
• It is the purest crystalline allotrope of carbon. It has a number of carbons, linked together tetrahedrally. Each tetrahedral unit consists of carbon bonded to four carbon atoms which are in turn bonded to other carbons. This gives rise to an allotrope of carbon having a three-dimensional arrangement of C-atoms.
• Each carbon is sp3 hybridized and forms covalent bonds with four other carbon atoms at the corners of the tetrahedral structure.
Physical Properties of Diamond
- It is extremely hard
- It has a very high melting point
- It has a high relative density
- It is transparent to X-rays
- It has a high value of the refractive index
- It is a bad conductor of electricity
- It is a good conductor of heat
- It is insoluble in all solvents
Other Carbon Allotropes
Buckminsterfullerene
• Buckminsterfullerene (C60) is also one of the allotropes of carbon. The structure of fullerene is like in a cage shape due to which it looks like a football.
Fullerenes
• They are spheroidal molecules having the composition, C2n, where n ≥ 30. These carbon allotropes can be prepared by evaporating graphite with a laser.
• Unlike diamond, fullerenes dissolve in organic solvents. The fullerene C60 is called ‘Buckminster Fullerene’. The carbon atoms are sp2 hybridized.
Silicates
• Fusing alkali oxides with SiO2 gives silicates. They contain discrete tetrahedral units. Silicon is sp3 hybridized. These allotropes of carbon are classified based on their structures.
1. Orthosilicates: They contain discrete SiO4 units. For example, Willemite ( ZrSiO4).
2. Pyrosilicate: Two units are linked together via an oxygen atom. The simplest ion of this type is Si2O76-. For example, Thortveite ( Sc2[Si2O7]).
3. Cyclic Silicates: The units share two oxygen atoms. Only two ions are known as of now, Si3O96- and Si6O1812-. For example, Beryl – Be3Al2Si6O18.
4. Chain Silicates: The linking of the units linearly results in the formation of chain silicates. They are of two types:
Metasilicates: Each tetrahedral unit shares two oxygen atoms to form a single chain carbon allotrope. For example, Spodumene NaAl(SiO3)2.
Amphiboles: When two linear chains are linked together, it results in amphiboles carbon allotrope. The parallel chains are held by sharing the oxygen atoms. For example, Asbestos: CaMg3O(Si4O11).
5. Two-dimensional silicates: Sharing of three oxygen atoms results in the formation of a two-dimensional silicate. For example, mica.
6. Three-dimensional silicate: When all the oxygen atoms are shared, it results in a three-dimensional network. For example, Zeolites.
pH SCALE – CONCEPT AND IMPORTANCE
• In 1909 S.P.L Sorenson, a Danish biochemist devised a scale known as pH to represents the H+ ion concentration of an aqueous solution. The pH value of any solution is a number that simply represents the acidity and basicity of the solution. The pH value of any solution is numerically equal to the logarithm of the inverse of the hydrogen ion (H+) concentration. Hence, the pH solution is referred to as the negative logarithm of hydrogen ion.
• pH stands for ‘potential of Hydrogen’ which measures the acidity or alkalinity of water-soluble substances. It is measured with a logarithmic scale known as pH.
• An acid is a substance that donates hydrogen ions i.e. when in a solution there are more hydrogen ions than hydroxide ions, the solution will be acidic.
• A base is a substance that accepts hydrogen ions i.e. when in a solution there are more hydroxide ions than hydrogen ions, the solution will be alkaline.
• The pH value of any solution is numerically equal to the logarithm of the inverse of the hydrogen ion (H+) concentration. Hence, the pH solution is referred to as the negative logarithm of hydrogen ion.
pH = -log [H+]
= log 1/ [H+]
pH of Neutral Solution (Pure Water):
• pH of water is 7. Whenever the pH of a solution is 7, it will be a neutral solution. Such a solution will have no effect on any litmus solution or any other indicator.
pH of an Acidic Solution:
• All the acidic solutions have a pH of less than 7. So, whenever a solution has a pH less than 7, it will be acidic in nature and it will turn blue litmus into the red as well as methyl orange pink and phenolphthalein colourless.
pH of a basic solution:
• All the alkaline solution has a pH of more than 7. So, whenever a solution has more than 7 values then it will be basic in nature and it will turn red litmus to blue, methyl orange to yellow and phenolphthalein to pink.
pH values of the common substance from our daily life
Solution pH
Conic HCl 0
Oil HCl 1.0
Gastric Juice 1.4
Lemon Juice 2.5
Vinegar 4.0
Tomato Juice 4.1
Coffee 5.0
Soft Drink 6.0
Milk 6.5
Pure Water 7.0
Saliva (before meal) 7.4
Saliva (after meal) 5.8
Blood 7.4
Eggs 7.8
Toothpaste 8.0
Baking Soda Solution 8.5
Washing Soda Solution 9.0
Milk of Magnesia 10.5
Household Ammonia 11.6
Dilute Sodium Hydroxide 13.0
Concentrated Sodium Hydroxide 14
Importance of pH
In Agriculture:
• By determining the pH of the soil. We can find whether it is acidic or alkaline. This helps in deciding the type of fertilizer to be used and the types of crops to sown.
In Biological process:
• By knowing pH we can adjust the medium of biological processes like fermentation, enzyme hydrolysis, sterilization etc.
In corrosion research:
• By measuring the pH of sea-water, the effect of alkaline sea-water on the material used for building ships and submarines is studied.
Buffer Solution
• A Buffer Solution or a buffer is defined as a solution whose pH does not change when small amount of an acid or base is added in it. It is used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications.
• Many life forms thrive only in a relatively small pH range so they utilize a buffer solution to maintain a constant pH.
• In nature, the bicarbonate buffering system is used to regulate the pH of blood. The solution of Sodium Acetete and Acetic acid is an example of an effective buffer solution.
CORROSION
• Corrosion can be defined as the process through which refined metals are converted into more stable compounds such as metal oxides, metal sulfides, or metal hydroxides.
• The rusting of iron involves the formation of iron oxides via the action of atmospheric moisture and oxygen.
• Corrosion is usually an undesirable phenomenon since it negatively affects the desirable properties of the metal.
• For example, iron is known to have good tensile strength and rigidity (especially alloyed with a few other elements). However, when subjected to rusting, iron objects become brittle, flaky, and structurally unsound.
• Corrosion can be classified as an electrochemical process since it usually involves redox reactions between the metal and certain atmospheric agents such as water, oxygen, sulphur dioxide, etc.
Definition
• Corrosion is defined as the natural process that causes the transformation of pure metals to undesirable substances when they react with substances like water or air.
• This reaction causes damage and disintegration of the metal starting from the portion of the metal exposed to the environment and spreading to the entire bulk of the metal.
Do All Metals Corrode?
• Metals placed higher in the reactivity series such as iron, zinc, etc. get corroded very easily and metals placed lower in the reactivity series like gold, platinum and palladium do not corrode.
• The explanation lies in the fact that corrosion involves oxidation of the metals. As we go down the reactivity series tendency to get oxidised is very low (oxidation potentials is very low).
Factors Affecting Corrosion
1. Exposure of the metals to air containing gases like CO2, SO2, SO3 etc.
2. Exposure of metals to moisture especially salt water (which increases the rate of corrosion).
3. Presence of impurities like salt (eg. NaCl).
4. Temperature: An increase in temperature increases corrosion.
5. Nature of the first layer of oxide formed: some oxides like Al2O3 forms an insoluble protecting layer which can prevent further corrosion. Others like rust easily crumble and expose the rest of the metal.
6. Presence of acid in the atmosphere: acids can easily accelerate the process of corrosion.
Types of Corrosion:
(i) Crevice Corrosion
• Whenever there is a difference in ionic concentration between any two local areas of a metal, a localized form of corrosion know as crevice corrosion can occur. Examples of areas where crevice corrosion can occur are gaskets, the undersurface of washers, and bolt heads.
• Example: All grades of aluminium alloys and stainless steels undergo crevice corrosion.
(ii) Stress Corrosion Cracking
• Stress Corrosion Cracking can be abbreviated to ‘SCC’ and refers to the cracking of the metal as a result of the corrosive environment and the tensile tress placed on the metal. It often occurs at high temperatures.
• Example: Stress corrosion cracking of austenitic stainless steel in chloride solution.
(iii) Intergranular Corrosion
• Intergranular corrosion occurs due to the presence of impurities in the grain boundaries that separate the grain formed during the solidification of the metal alloy. It can also occur via the depletion or enrichment of the alloy at these grain boundaries.
• Example: Aluminum-base alloys are affected by IGC.
(iv) Galvanic Corrosion
• When there exists an electric contact between two metals that are electrochemically dissimilar and are in an electrolytic environment, galvanic corrosion can arise. It refers to the degradation of one of these metals at a joint or at a junction. A good example of this type of corrosion would be the degradation that occurs when copper, in a salt-water environment, comes in contact with steel.
• Example: When aluminium and carbon steel are connected and immersed in seawater, aluminium corrodes faster and steel is protected.
(iv) Pitting Corrosion
• Pitting Corrosion is very unpredictable and therefore is difficult to detect. It is considered one of the most dangerous types of corrosion. It occurs at a local point and proceeds with the formation of a corrosion cell surrounded by the normal metallic surface. Once this ‘Pit’ is formed, it continues to grow and can take various shapes. The pit slowly penetrates metal from the surface in a vertical direction, eventually leading to structural failure it left unchecked.
• Example: Consider a droplet of water on a steel surface, pitting will initiate at the centre of the water droplet (anodic site).
(v) Uniform Corrosion
• This is considered the most common form of corrosion wherein an attack on the surface of the metal is executed by the atmosphere. The extent of the corrosion is easily discernible. This type of corrosion has a relatively low impact on the performance of the material.
• Example: A piece of zinc and steel immersed in diluted sulphuric acid would usually dissolve over its entire surface at a constant rate.
Prevention of Corrosion
• Preventing corrosion is of utmost importance in order to avoid huge losses. Majority of the structures we use are made out of metals. This includes bridges, automobiles, machinery, household goods like window grill, doors, railway lines, etc.
• There are various methods to prevent corrosion. These include,
1. Electroplating
• It is the process by which a metal (I) is coated with a thin layer of another metal (II) using electrolysis. In this way, the new metal coating protects the metal (I) from corrosion.
• In the process, a metal is used as the coating, (metal II) is kept as the anode and metal (I) (metal to be plated) is kept as the cathode, i.e. metal I is connected to the negative terminal and metal II is connected to the positive terminal. These 2 electrodes are immersed in an electrolyte and when power is supplied, oxidation happens in the anode, thereby dissolving metal II ions in the electrolyte. These dissolved metal II ions are reduced at the cathode thereby providing a coating on metal I.
• The metals most commonly used as the anode are Copper, Nickel, Gold, Silver, Zinc, etc.
2. Cathodic Protection
• In this process, the base metal is connected to a sacrificial metal that corrodes instead of the base metal.
• By doing so this sacrificial metal (which is more reactive than the base metal) will give out electrons and get oxidised. The ions thus formed takes part in the corrosion reactions thereby saving the base metal.
3. Galvanization
• This process involves coating iron with a thin layer of zinc. It is generally done by dipping iron in molten zinc. The zinc layer coating thus protect the iron inside from corrosion
4. Painting and Greasing
• Providing a layer of paint or grease on the metal can prevent the exposure of the metal with the external environment thereby preventing corrosion.
5. Choosing the right material
• Eg: Aluminium and stainless steel are highly corrosion resistant
6. Using corrosion Inhibitor
• Corrosion inhibitors are chemicals which when added to the corrosion environment has the ability to cut down the rate of corrosion.
CATALYSTS
• Catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction.
• Most of the time a catalyst is used to speed up or increase the rate of the reaction.
• However, if we go into a more deeper level, catalysts are used to break or rebuild the chemical bonds between the atoms which are present in the molecules of different elements or compounds.
• In essence, catalysts encourage molecules to react and make the whole reaction process easier and efficient.
Important characteristic features of catalysts:
• A catalyst does not initiate a chemical reaction.
• A catalyst is not consumed in the reaction.
• Catalysts tend to react with reactants to form intermediates and at the same time facilitate the production of the final reaction product. After the whole process, a catalyst can regenerate.
• A catalyst can be either solid, liquid or gaseous. Some of the solid catalysts include metals or their oxides, including sulfides, and halides. Semi-metallic elements such as boron, aluminium, and silicon are also used as catalysts. Likewise, liquid and gaseous elements which are in pure form are used as catalysts. Sometimes, these elements are also used along with suitable solvents or carriers.
• The reaction which involves a catalyst in their system are known as catalytic reactions.
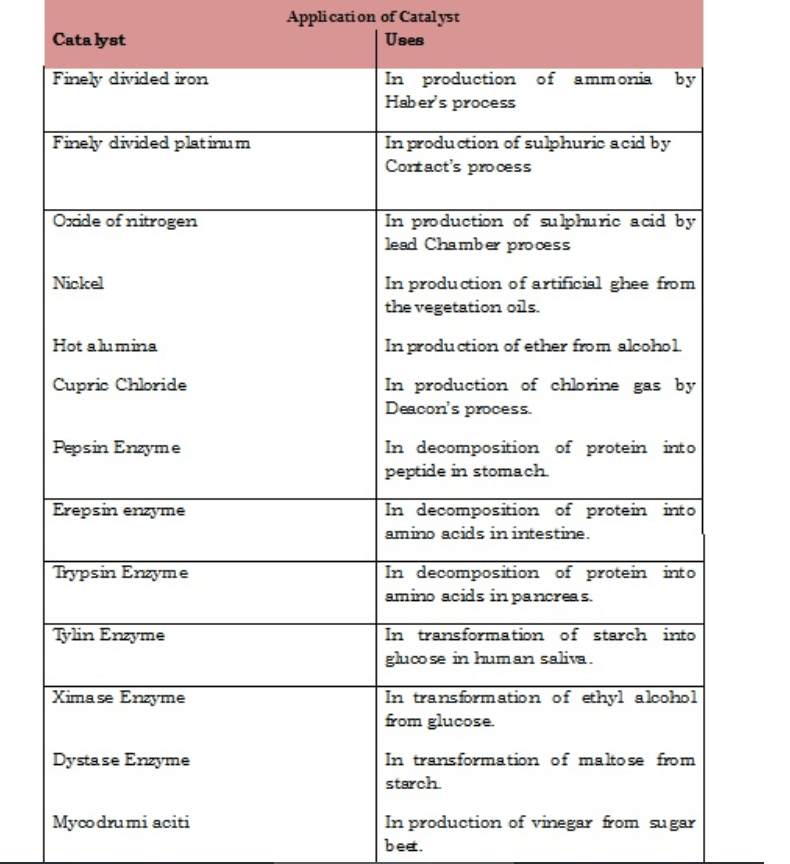
Types of Catalysts with Examples
• There are several types of catalysts that can be used depending on the need or requirement of the chemical reaction. They are as follows;
Positive Catalysts
• Catalysts which increase the rate of a chemical reaction are called positive catalysts. They increase the rate of reaction by lowering the activation energy barriers such that a large number of reaction molecules are converted into products, thereby the percentage of yield of products increases.
• Example: In the preparation of NH3 by Haber’s process Iron oxide acts as a positive catalyst and increases the yield of ammonia in spite of less reaction of Nitrogen.
Negative Catalysts
• Catalysts which decrease the rate of reaction are called negative catalysts. They decrease the rate of reaction by increasing the activation energy barrier which decreases the number of reactant molecules to transform into products thereby decreasing the rate of reaction.
• Example: Decomposition of Hydrogen peroxide into water and oxygen is retarded by using Acetanilide, which acts as a negative catalyst and decreases the rate of decomposition of hydrogen peroxide.
Auto catalysts
• When any product forms during any chemical- reaction act like the catalyst for the same chemical reaction then it is called an auto catalyst
Induced catalysts
• When the product of any chemical reaction acts like the catalyst of any other chemical reaction then it is called induced catalyst
Catalysis
• When a catalyst is used to increase the rate of a chemical reaction this phenomenon is known as catalysis.
Types of Catalysis
• On the basis of nature and the physical state of substance employed in the chemical reaction, catalysis is of three types;
- Homogeneous catalysis
- Heterogeneous catalysis
- Autocatalysis
Heterogeneous Catalysis
• In this type of catalysis, the reacting substances in a reaction and catalyst employed in that reaction are not in the same state of matter.
Homogeneous Catalysis
• The catalysis in which the catalyst employed in the reaction and the reactants are in the same state of matter, that process is referred to as homogeneous catalysis.
Autocatalysis
• In the autocatalytic reaction, there is no specific catalyst that is added. Instead, one of the products acts as a catalyst and increases the rate of formation of products.
Enzymes
• The compressed condensed nitrogenous substance found to be in microbes or microorganism through which the process of fermentation is performed is called an enzyme. This is present in the cells of every living creature and it is responsible and fully accountable for the various metabolic activities taking place in the living beings.
• Enzymes are sometimes called biochemical catalysts. Thus enzymes are condensed substances like protein and these act like contact catalysts and provoke the decomposition process to complex high organic substances into a simpler substance.
• The enzymes are very exclusive and they perform a single activity of the time. The activities of enzymes are abruptly diminished or vanished in the presence of any toxic substance at high temperature. At ordinary temperature, enzyme functions optimally.
SOAPS AND DETERGENTS
Soaps
• A soap is a water-soluble compound which is made via a process called saponification by the reaction between sodium hydroxide or potassium hydroxide with vegetable or animal oil (fats).
Characteristics of Soap
Hardness – Harder soap which is a dense bar lasts longer.
Cleansing – The first reason the majority of people use soap is to get clean. A soap molecule consists of a chain of carbon atoms where one end of the chain attracts oil and the other attracts water. Soap should be balanced and not too much or too less of cleansing ingredient should be added.
Conditioner – Soap conditioners are referred to as emollients. Once you have washed your hands and what’s left behind on your skin after you rinse, depends on the type of soap a person uses. For instance, consider a person with dry skin, he/she should select a soap with moisturizing emollients that can prevent water evaporation.
Lather – Most people like soap which produces lather. The balance of bubbles and cleansing, soothing cream makes lather so satisfying.
Fragrance – It is an essential factor. Aromas evoke a unique combination of personal memory and enrich our daily life. Fragrances revitalize us, calm us, and most importantly mask our body odors.
Preparation of Soap
• The most commonly used soap making process is the saponification of oils and fats.
• This process involves heating oils and fats reacting them with a liquid alkali to produce soap plus water plus glycerine.
Saponification
• The other soapmaking process is with the neutralization of fatty acids with an alkali. Oils and fats are hydrolyzed with high-pressure steam to yield glycerine and crude fatty acids.
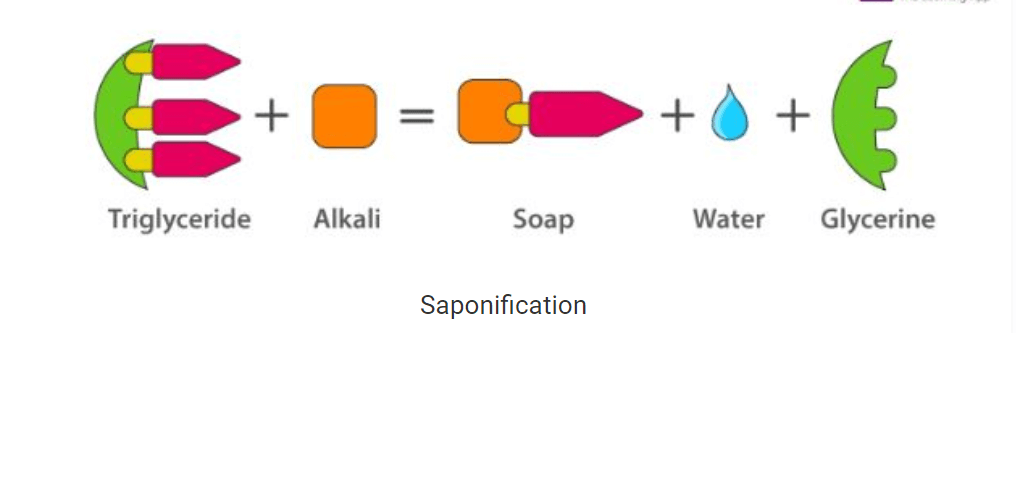
• The fatty acids are later purified by the method of distillation and neutralized with an alkali to produce water and soap.

Detergents
• Amphipathic molecules that contain charged hydrophilic or polar groups at the end of long lipophilic hydrocarbon groups are called detergents.
• The charged hydrophilic group is also called the head and the long lipophilic hydrocarbon group is called the tail.
• Detergents are also known as surfactants as they have the ability to decrease the surface tension of water.
Properties of Detergents
• The concentration at which micelles formation starts is called as critical micelle concentration (CMC).
• Aggregation number is the average number of monomers in a micelle.
• Relative micelle size is indicated by micelle molecular weight.
• The temperature at which the detergent solution is around or above its critical micelle concentration separates into two phases is called the cloud point.
Cleansing Action of Soaps and Detergents
• Most of the dirt is oily in nature and oil does not dissolve in water. The molecule of soap constitutes sodium or potassium salts of long-chain carboxylic acids. In the case of soaps, the carbon chain dissolves in oil and the ionic end dissolves in water. Thus the soap molecules form structures called micelles.
• In micelles, one end is towards the oil droplet and the other end which is the ionic faces outside. Therefore, it forms an emulsion in water and helps in dissolving the dirt when we wash our clothes.
• Soap is a kind of molecule in which both the ends have different properties.
- Hydrophilic end
- Hydrophobic end
• The first one is the hydrophilic end which dissolves water and is attracted to it whereas the second one is the hydrophobic end that is dissolved in hydrocarbons and is water repulsive in nature.
• If on the surface of the water, soap is present then the hydrophobic tail which is not soluble in water will align along the water surface.
How do Soaps and Detergents Clean out Dirt?
• Cleaning a soiled surface is a four step process. In the first step, the surface to be cleaned is made wet with water. In the second step, soap or detergent is applied to the surface to be absorbed.
• Soaps and detergents are also called surface active agents, or surfactants. Surface active molecules present in soaps and detergents dissolve in water. This solution serves to loosen surface tension, or the force that holds together molecules on a surface or on cloth. When this happens, it helps water to spread easily over a surface or soak into clothes.
• In the third step, when clothes are rubbed together, either by hand or in a washing machine, dirt particles are broken up as surface active molecules work to separate the dirt from clothes and deposit them in the water.
• In the fourth and final step of the cleaning process, the separated dirt is prevented from going and re-depositing on the surface of clean clothes. Dirt particles are coated with soap and detergent molecules. This keeps them suspended in water until the dirt is washed away with rinsing
Micelles
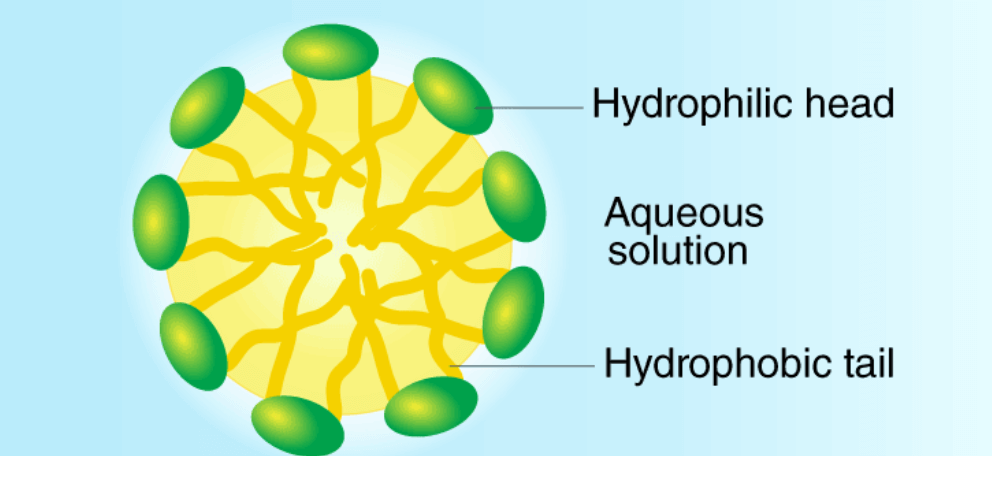
• In water, the soap molecule is uniquely oriented which helps to keep the hydrocarbon part outside the water.
• When the clusters of molecules are formed then hydrophobic tail comes at the interior of the cluster and the ionic end comes at the surface of the cluster and this formation is called a micelle.
• When the soap is in the form of micelles then it has the ability to clean the oily dirt which gets accumulated at the center.
• These micelles remain as colloidal solutions. Therefore the dirt from the cloth is easily washed away.
• The soap solution appears cloudy as it forms a colloidal solution which scatters light.

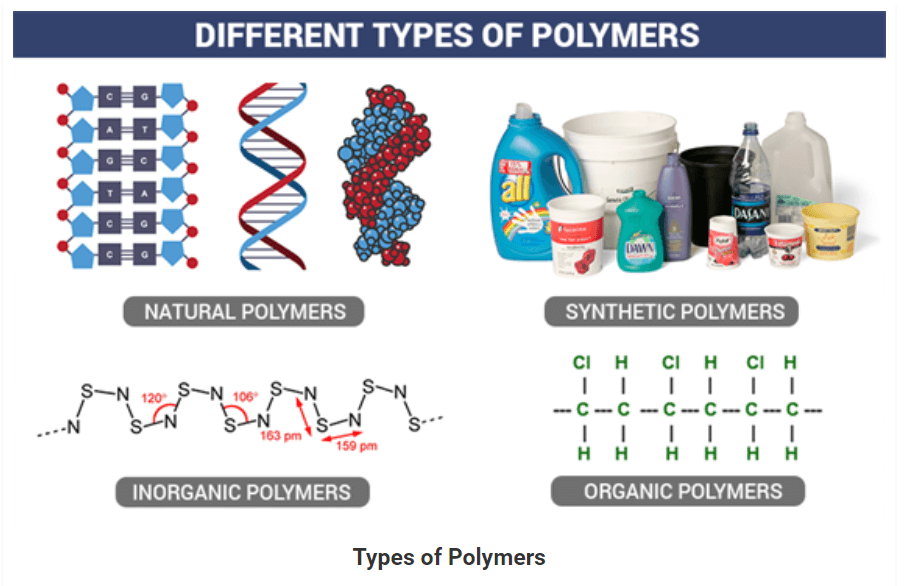
POLYMERS
• A polymer is a large molecule or a macromolecule which essentially is a combination of many subunits. The term polymer in Greek means ‘many parts’. Polymers can be found all around us. From the strand of our DNA which is a naturally occurring biopolymer to polypropylene which is used throughout the world as plastic.
• Polymers may be naturally found in plants and animals (natural polymers) or may be man-made (synthetic polymers). Different polymers have a number of unique physical and chemical properties due to which they find usage in everyday life.
• Polymers are all created by the process of polymerization wherein their constituent elements called monomers, are reacted together to form polymer chains i.e 3-dimensional networks forming the polymer bonds.
• The type of polymerization mechanism used depends on the type of functional groups attached to the reactants. In biological contexts, almost all macromolecules are either completely polymeric or are made up of large polymeric chains.
Classification of Polymers
• Polymers cannot be classified under one category because of their complex structures, different behaviours, and vast applications. We can, therefore, classify polymers based on the following considerations.
Classification of Polymers based on the Source of Availability
• There are three types of classification under this category, namely, Natural, Synthetic, and Semi-synthetic Polymers.
Natural Polymers:
• They occur naturally and are found in plants and animals. For example proteins, starch, cellulose and rubber. To add up, we also have biodegradable polymers which are called biopolymers.
Semi-synthetic Polymers:
• They are derived from naturally occurring polymers and undergo further chemical modification. For example, cellulose nitrate, cellulose acetate.
Synthetic Polymers:
• These are man-made polymers. Plastic is the most common and widely used synthetic polymer. It is used in industries and various dairy products. For example, nylon-6, polyethers etc.
Classification of Polymers based on the Structure of the Monomer Chain
• This category has the following classifications:
Linear Polymers
• The structure of polymers containing long and straight chains fall into this category. PVC, i.e. poly-vinyl chloride is largely used for making pipes and electric cables is an example of a linear polymer.
Branched-chain Polymers
• When linear chains of a polymer form branches, then, such polymers are categorized as branched chain polymers. For example, Low-density polythene.
Cross-linked Polymers
• They are composed of bifunctional and trifunctional monomers. They have a stronger covalent bond in comparison to other linear polymers. Bakelite and melamine are examples in this category.
Classification Based on Molecular Forces
Elastomers: These are rubber-like solids weak interaction forces are present. For example, Rubber.
Fibres: Strong, tough, high tensile strength and strong forces of interaction are present. For example, nylon -6, 6.
Thermoplastics: These have intermediate forces of attraction. For example, polyvinyl chloride.
Thermosetting polymers: These polymers greatly improve the material’s mechanical properties. It provides enhanced chemical and heat resistance. For example, phenolics, epoxies, and silicones.
Structure of Polymers
• Most of the polymers around us are made up of a hydrocarbon backbone. A hydrocarbon backbone being a long chain of linked carbon and hydrogen atoms, is possible due to the tetravalent nature of carbon.
• A few examples of a hydrocarbon backbone polymer are polypropylene, polybutylene, polystyrene. Also, there are polymers which instead of carbon have other elements in its backbone. For example, Nylon, which contains nitrogen atoms in the repeated unit backbone.
Types of Polymers
On the basis of the type of the backbone chain, polymers can be divided into:
- Organic Polymers: Carbon backbone.
- Inorganic Polymers: Backbone constituted by elements other than carbon.
Biodegradable Polymers
• The polymers which are degraded and decayed by microorganisms like bacteria are known as biodegradable polymers. These types of polymers are used in surgical bandages, capsule coatings and in surgery. For example, Poly (3-hydroxybutyrate-co-3-hydroxyvalerate) [PHBV]
High-Temperature Polymers
• These polymers are stable at high temperatures. Due to their high molecular weight, these are not destroyed even at very high temperatures. They are extensively used in the healthcare industries, for making sterilization equipment and in the manufacturing of heat and shock-resistant objects.
Few of the important polymers are:
Polypropylene
• It is a type of polymer that softens beyond a specific temperature allowing it to be moulded and on cooling it solidifies. Due to its ability to be easily moulded into various shapes, it has a lot of applications.
• A few of which are in stationary equipment’s, automotive components, reusable containers speakers and much more. Due to its relatively low energy surface, the polymer is fused with the welding process and not using glue.
Polyethene
• It is the most common type of plastic found around us. Mostly used in packaging from plastic bags to plastic bottles. There are different types of polyethene but their common formula being (C2H4)n.
Properties of Polymers
Physical Properties
• As chain length and cross-linking increases the tensile strength of the polymer increases.
• Polymers do not melt; they change state from crystalline to semi-crystalline.
Chemical Properties
• Compared to conventional molecules with different side molecules, the polymer is enabled with hydrogen bonding and ionic bonding resulting in better cross-linking strength.
• Dipole-dipole bonding side chains enable the polymer for high flexibility.
• Polymers with Van der Waals forces linking chains are known to be weak, but give the polymer a low melting point.
Optical Properties
• Due to their ability to change their refractive index with temperature as in the case of PMMA and HEMA: MMA, they are used in lasers for applications in spectroscopy and analytical applications.
Some Polymers and their Monomers
- Polypropene, also known as polypropylene, is made up of monomer propene.
- Polystyrene is an aromatic polymer, naturally transparent, made up of monomer styrene.
- Polyvinyl chloride (PVC) is a plastic polymer made of monomer vinyl chloride.
- The urea-formaldehyde resin is a non-transparent plastic obtained by heating formaldehyde and urea.
- Glyptal is made up of monomers ethylene glycol and phthalic acid.
- Bakelite is a plastic which is made up of monomers phenol and aldehyde.
Uses of Polymers
• Polypropene finds usage in a broad range of industries such as textiles, packaging, stationery, plastics, aircraft, construction, rope, toys, etc.
• Polystyrene is one of the most common plastic, actively used in the packaging industry. Bottles, toys, containers, trays, disposable glasses and plates, tv cabinets and lids are some of the daily-used products made up of polystyrene. It is also used as an insulator.
• The most important use of polyvinyl chloride is the manufacture of sewage pipes. It is also used as an insulator in the electric cables.
• Polyvinyl chloride is used in clothing and furniture and has recently become popular for the construction of doors and windows as well. It is also used in vinyl flooring.
• Urea-formaldehyde resins are used for making adhesives, moulds, laminated sheets, unbreakable containers, etc.
• Glyptal is used for making paints, coatings, and lacquers.
• Bakelite is used for making electrical switches, kitchen products, toys, jewellery, firearms, insulators, computer discs, etc.
Commercial Uses of Polymers

Vulcanization of rubber
• Natural rubber is highly elastic to be of poor physical stability.
Addition of 5% sulphur, enhances the crosslinking of the linear chains and thus improves the stiffening of the rubber for an application like vehicle tires.
List of Some common man-made Polymers and their Uses:
1. Polythene
• Packaging, material, carry bags, bottles.
2. Teflon
• Nonstick Kitchen ware
3. Polypropene
• Bottles, Crates
4. Melamine
• Crockery
5. Polyvinyl chloride (PVC)
• Pipes Insulation
6. Lexan
• Bullet proof glass
7. Vinyl rubber
• Rubber erasers
8. Bakelite
• Electrical insulation buttons
9. Polystyrene
• Foam Thermocole
10. Poly (Styrene butadiene)
• Rubber bubble gum
11. Nylon (Polyester)
• Fibres, ropes
12. Luminous Paints
• Glow when exposed to light. They are applied on a surface to protect it from corrosion and weathering.
13. Antimicrobial polymers (polymeric biocide)
• The ability to inhibit the growth of microorganisms
14. Antigen
• Substance capable of stimulating formation of antibodies.
15. Antipyretie
• A substance used to lower body temperature.
16. Pesticides
• Used to kill animals
17. Para-aramid fibre (Kevlar)
• Manufacturing armour, sports and musical equipment. Used in the field of cryogenics.
18. Polyacrylonitrile (PAN) (Orlon)
• Used for making clothes and fabrics like sweaters, hats,rugs, etc
19. Copolyamid (Technora)
• Used for manufacturing optical fiber cables, drumheads, automotive industry, ropes, wire ropes and cables.
20. Polytetrafluoroethylene (PTFE)(Viton)
• Viton B is used in chemical process plants and gaskets. As it depends upon the grade of the polymer
